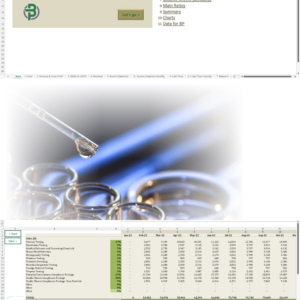
| Population (m) | 24.6 | |
| GDP (US$ t) | 1.3 | |
| Total Healthcare Expenditure (US$ b, est.) | 133.4 | |
| Total Beauty & Wellbeing Expenditure (US$ b, est.) | 172.2 | |
| Alcohol Consumption (Litres Per Capita Per Year) | 10.6 | |
| Prevalence of Cannabis Users (%) | 10.2 | |
| Source: World Bank/ ABS/ WHO/ UNODC/ Prohibition Partners | ||
In June 2015, the Lambert family donated $33.7 million to the University of Sydney, establishing the ‘Lambert Initiative’. This is the largest Australian medicinal cannabis research group, providing an invaluable boost to the development of treatments for childhood epilepsy, cancer, chronic pain, obesity, anorexia and dementia.
A 2016 University of Sydney report, Medicinal Cannabis in Australia: Science, Regulation & Industry, found that the Australian medicinal cannabis market, once opened up, could spark initial demand for as much as 8,000 kg of product.
Australia Cannabis Legalization
The Narcotic Drugs Amendment Bill was passed by the Australian Federal Government in February 2016, a milestone for medicinal cannabis in the country. In October 2016, the Narcotic Drugs Regulation 2016 was published, which adds a lot more detail. Several States and Territories have since amended and/or introduced new legislation and regulation.
In January 2018, Australia said it planned to become the fourth country in the world to legalize medicinal cannabis exports in a bid to score a piece of the estimated $57 billion global market. The export of medicinal cannabis products was legalized in February 2018 through the Narcotic Drugs Amendment (Cannabis) Regulations 2018.
Provided domestic supply of medicinal cannabis is not affected, the following products are eligible for export if granted a license and permit to export:
- Medicinal cannabis products manufactured in Australia under a GMP license.
- Medicinal cannabis products listed as export-only, or registered, on the Australian Register of Therapeutic Goods (ARTG)
- Extracts of cannabis (or extracts of cannabis resin) manufactured under a Narcotic Drugs Act 1967 license and permit that are not in the final dosage form.
Amendments to the Narcotic Drugs Act 1967 (ND Act), which came into effect on 24 December 2021, introduced a single license model for regulating medicinal cannabis cultivation, production and/or manufacture activities. A medicinal cannabis license can authorize any or all of the following activities for medicinal or scientific purposes:
- cultivation and/or production of cannabis plants, cannabis or cannabis resin
- manufacture of a cannabis drug for one or more permitted supplies.
In September 2019, the Australian Capital Territory became the first state or territory of Australia to legalize recreational use of cannabis. Since 31 January 2020 residents have been allowed to grow two plants and possess 50 g, though sales or other transfer is prohibited, including cannabis seeds. Federal law also remains enforceable.
Australian Cannabis Market
As of January 2024, a total of 127 licenses were issued under the scheme: 48 medicinal cannabis licenses (commercial cultivation and production), 44 cannabis manufacture licenses and 35 importer licenses.
The Therapeutic Goods Administration (TGA) has approved Special Access Scheme (SAS). The SAS allows prescribers (including nurse practitioners) to prescribe medicinal cannabis products for a single patient on a case-by-case basis. Chronic pain and anxiety are by far the most common conditions authorized for treatment by a medical cannabis product (50% and 25% of applications respectively), the other leading conditions approved for treatment include sleep disorder, cancer pain and symptom management, post-traumatic stress disorder, insomnia, depression and neuropathic pain. Those conditions represent more than 90% of all SAS B prescriptions for medical cannabis. Through Dec. 31, 2023, doctors prescribed treatment for more than 200 different conditions.
Patient access is also available via the Authorised Prescriber (AP) pathway. The AP pathway allows authorised medical practitioners to supply therapeutic goods (such as medicines or medical devices) that are not included in the Australian Register of Therapeutic Goods (ARTG) to patients with a particular medical condition without the need for individual patient approvals. As of December 2023, there were 2,482 medicinal cannabis Authorised Prescribers in Australia and 963,413 patients reported by Authorised Prescribers to the TGA.
TGA data shows that since its legalization, a total of 1.4 million prescriptions for the drug have been issued – either through authorized prescribers or through the special access scheme. In 2016-2018, around 3,384 patients accessed medical cannabis, rising to 25,973 in 2019, 75,156 patients in 2020, 272,687 and 443,685 new patients in 2021 and 2022, and reaching over 600,000 patients in 2023.
North Sydney-based data firm FreshLeaf Analytics estimated the Australian medical cannabis market increased to about 95 million Australian dollars in product sales in 2020 from $30 million in 2019. Based on the Penington Institute report “Cannabis in Australia 2023″, Australians spent approximately $234 million during the 2022 calendar year, and approximately $210 million between January and June 2023.
Australia Cannabis Forecast
Cannabis Jobs Australia estimates there will be 50,000 cannabis jobs in Australia by 2028. Australia’s legal cannabis market is forecast to grow from $52 million in 2018 to $1.2 billion in 2027, the 5th largest in the world, according to the report “The Road Map to a $57 Billion Worldwide Market”.
In the Oceania Cannabis Report – produced by London-based advisory group Prohibition Partners – Australian cannabis market only in medical part is estimated to be worth up to $1.3b by 2028 while recreational part is about $3.5b.
Recreational Cannabis Legalization In Australia
In 2023, the Legalize Cannabis Party introduced draft bills in the Victorian, New South Wales and WA state parliaments to legalize the recreational use of cannabis, allowing possession of up to 50g and six plants per household. The bill also proposes a national cannabis licensing scheme similar to the Canadian model, where cannabis can be sold by both government-run dispensaries and licensed private sellers and the regulator oversees small-scale commercial production and sales operations, acting as a wholesaler between producers and retailers, setting a wholesale price.
Australian Cannabis Market Infographics
Cannabis Cultivation Business Plan Sample, Australia
'70% ready to go' business plan templates
Our cannabis financial models and cannabis business plan templates will help you estimate how much it costs to start and operate your own cannabis business, to build all revenue and cost line-items monthly over a flexible seven year period, and then summarize the monthly results into quarters and years for an easy view into the various time periods. We also offer investor pitch deck templates.
Best Selling Templates
Hemp CBD business plan templates are available at hempcbdbusinessplans.com.